8.4. Creating and plotting VPaths¶
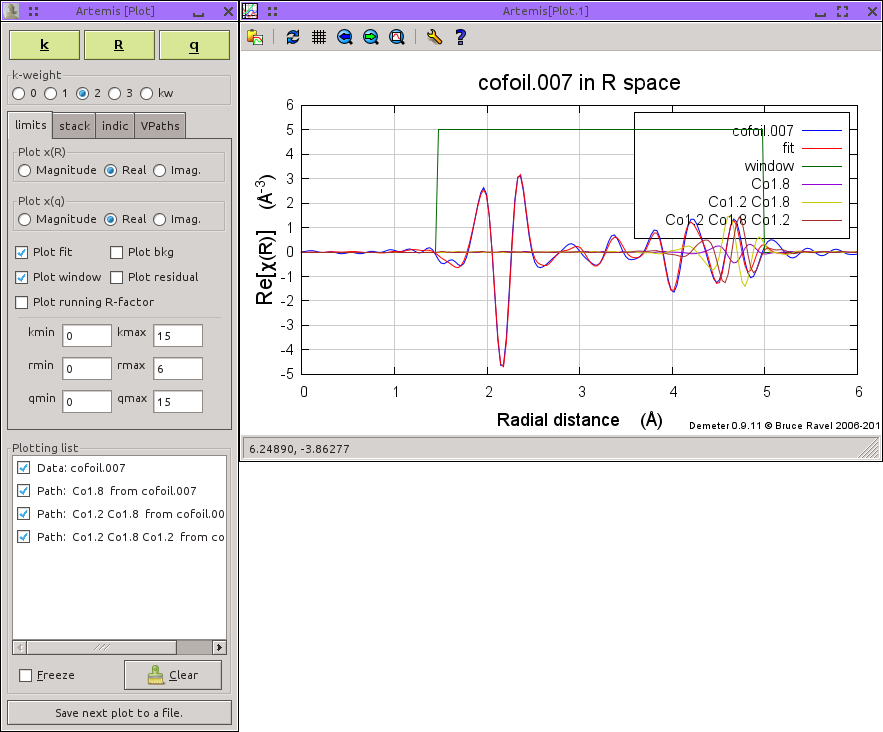
A common problem with visualizing the paths that contribute to a fit occurs when several single and multiple scatteriung paths contribute spectral wieght in the same region of R space. In a well-ordered material like these data on a cobalt foil, a distant single scattering path may be of the same length as two or more important collinear multiple scattering paths. The fit involves a subtle superposition of all the paths in the fit. This single scattering path and its related multiple scattering paths all contribute spectral weight in the same region of R-space.
Plotting them together results in a complicated figure with lots of overlapping traces. It is hard to see how each path contributes to the fit. Indeed, it is hard to ascertain much beyond the fact that these three paths have a complicated phase relationship between them.
ARTEMIS offers a handy visualization tool called a virtual path, or VPath. A VPath is simply a group of paths which are added together after being evaluated in a fit. The sum of these paths is then plotted as a single item in a fit. Here is the plot made with the VPath constructed from the three paths in the previous figure. While this plot hides the individual contributions of the three path, it shows how those three affect the fit when added together.
The VPath is made by marking the contributing paths, then choosing . You will be prompted for a name for the VPath. This name will be used to identify the VPath in a plot. The VPath is then inserted into the list of the VPaths tab.
Here is another example of how a VPath can help visualize the components of a fit. The data in the following figures are of uranium LIII edge EXAFS of uranyl acetate in solution. Uranyl compounds are notable for having two, very short double-bonded oxygen atoms in a trans configuration and at a distance of about 1.78 Å. The remaining oxygen atoms lie in an equatorial plane. These are single-bonded and at a distance of around 2.4 Å.
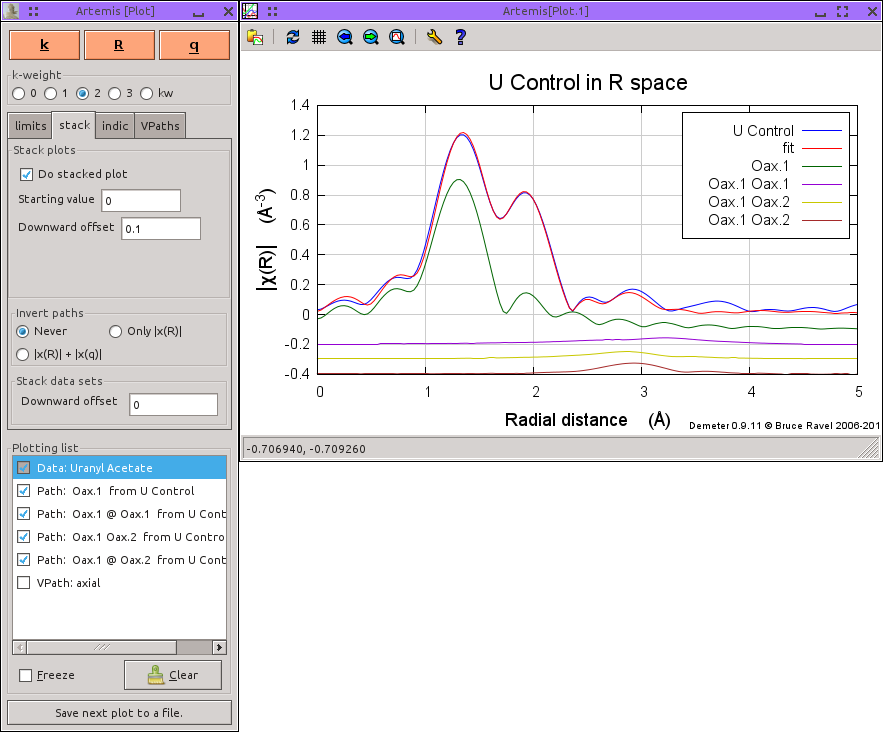
The two axial oxygen atoms usually have a rather small σ2 and contribute very strongly at low R in the Fourier transform. Because those oxygen atoms are at such a short distance, there are a few multiple scattering paths that contribute significant spectral weight just beyond the region dominated by single scattering from the equatorial oxygen atoms.
Here is the plot the fit to the uranyl acetate data along with each of the single and multiple scattering paths that involve the axial oxygen atoms. Again, it is hard to appreciate how the various multiple scattering paths contribute to the fit.
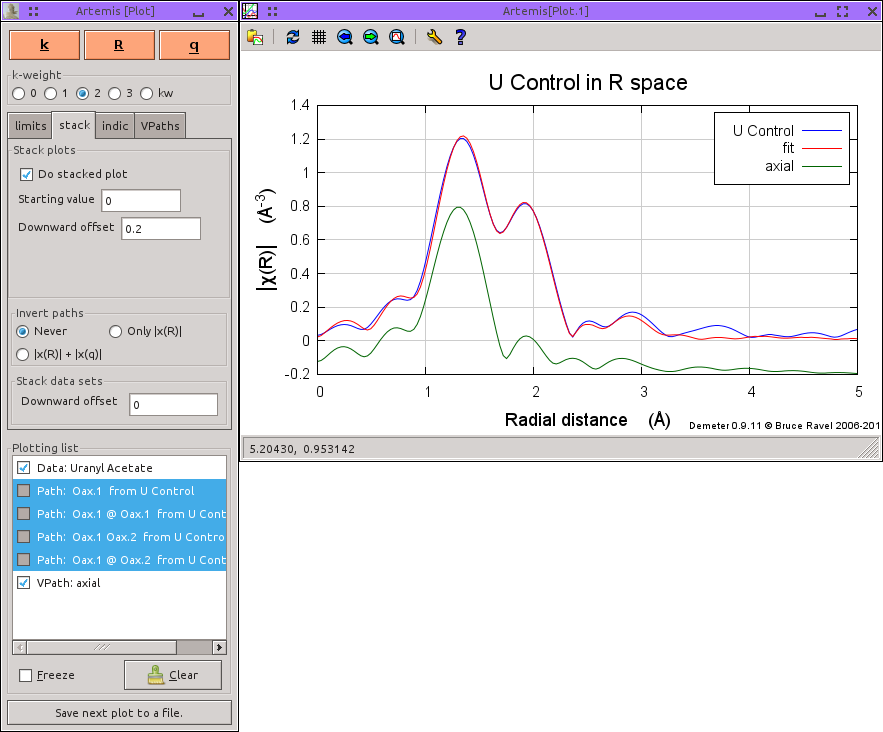
In this case, it is nice to combine all these paths into a VPath which represents the entire contribution to the EXAFS from the axial oxygen atoms.
DEMETER is copyright © 2009-2016 Bruce Ravel – This document is copyright © 2016 Bruce Ravel
This document is licensed under The Creative Commons Attribution-ShareAlike License.
If DEMETER and this document are useful to you, please consider supporting The Creative Commons.